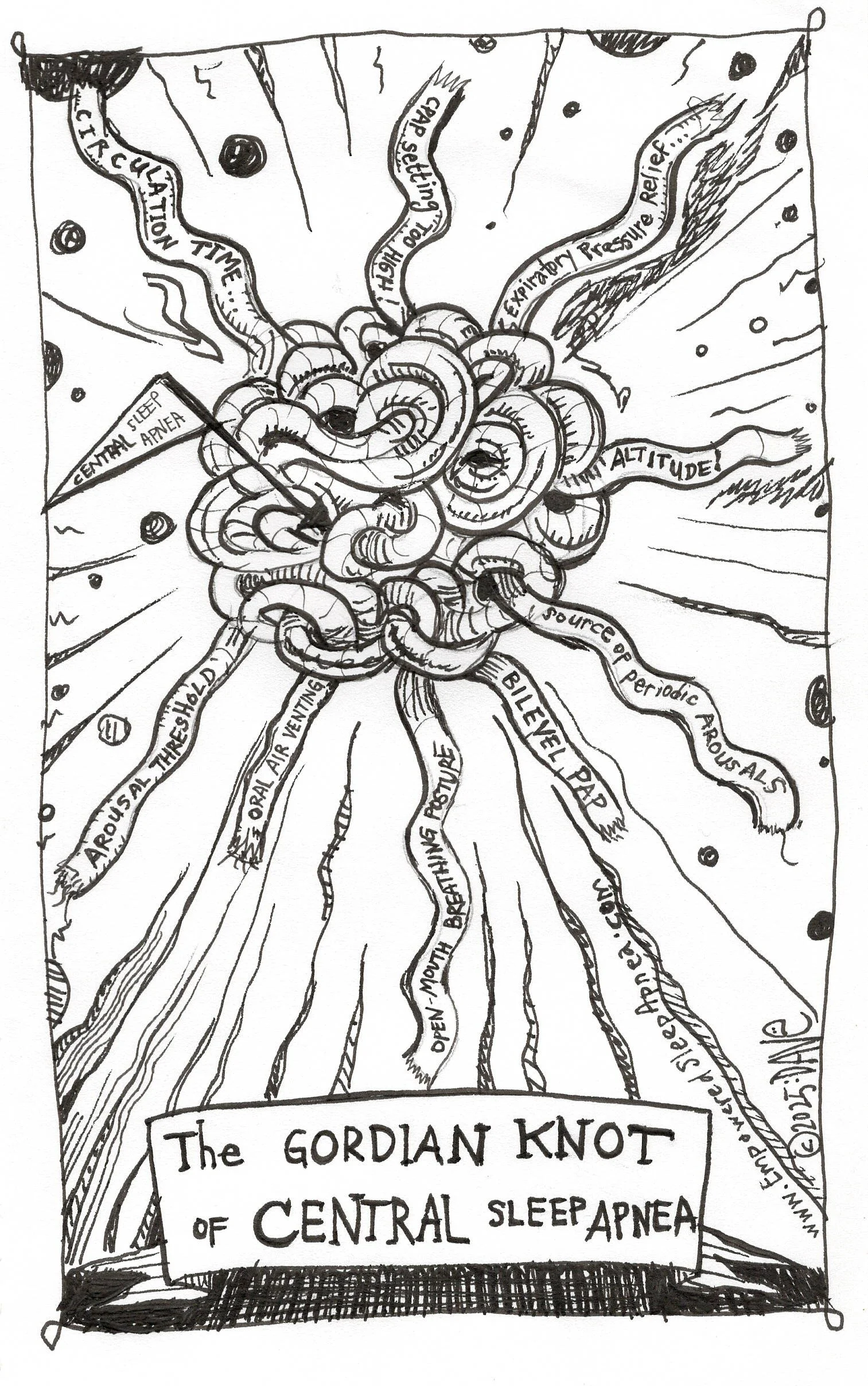
Unraveling the Gordian Knot of Central Sleep Apnea
OR: An EMPOWERED Response to the
2025 AASM Clinical Practice Guideline on Central Sleep Apnea
By David E McCarty MD FAASM (but you can call me Dave)
11 September 2025
~ ~ ~ ~ ~
“Never cut what you can untie.”
~ ~ ~ ~ ~
Unraveling a Gordian Knot requires nimble fingers and a keen eye! Care to have a go?
~ ~ ~ ~ ~
Introduction: Knots and Labels
Every field of medicine has its knots.
You know…the places where complexity gets tied up into a ball, where cause and effect loop back on themselves like a tangled fishing line, where multiple elements interact with each other in non-linear ways to produce a particular pattern of dis-ease.
The pattern of breathing that we all call “central sleep apnea” (CSA) is one of those knots.
Look: CSA is not so much a disorder as it is a pattern or behavior—an emergent phenomenon of physiology caught in feedback loops, oscillating between breath and no breath. Why does it happen? Well, it’s not so simple…
There are many moving parts, you see…
Yet in our eagerness to categorize & control, medical history demonstrates a long history of responding to Gordian Knots with labels. The labeling creates a sense of order. We place a tag on the bundle—Central Sleep Apnea, G47.31—and congratulate ourselves on our tidy taxonomy. Once a label is affixed, it makes sense to reach reflexively for the treatment that best pairs with that label, as if we were sommeliers choosing a chardonnay for the Sea Bass special. If the label is “treatment emergent central sleep apnea” (TECSA)? Then you must need ASV! You’ve met the criteria for opioid-associated CSA? Step right up for your BiPAP with a backup rate!
As I’ve noted previously, though, the labeling comes with hefty price: with the label comes a form of blindness…with the labelling, we forget about the places we can intervene!
In this piece, I’m going to take a hard look at the AASM’s updated Clinical Practice Guidelines for the management of CSA. We’ll talk about what’s changed since the last effort in 2012, and we’ll take a look at some of the limitations of this guidance, when it comes to boots-on-the-ground management of CSA.
As we’ll find out, there are some significant opportunities for increased clarity.
~ ~ ~ ~ ~
Updated Guidance for 2025
I’ll start with this: I’ve stated before that a major challenge we face in Sleep Medicine is that concepts of enormous complexity resist accurate encapsulation within a diagnostic label…and yet: diagnostic labels are a requirement within our current healthcare payor system. Having established this tension, I’ll start with the observation that the newly published 2025 AASM Clinical Practice Guideline on Central Sleep Apnea in Adults¹ is the latest chapter in this labeling tradition.
Kudos where kudos are due: this is a thoughtful, evidence-weighed, painstakingly constructed document. It organizes therapies by etiology, carefully weighing the level of evidence with the GRADE paradigm (Grading of Recommendations, Assessment, Development, and Evaluation), and acknowledges uncertainties with admirable transparency.
And yet, in practice, somehow this document risks perpetuating the same reflexes toward device-based strategies, a practice that’s defined the last decade of CSA management: see the label, match the device.
This essay is an attempt to untie the knot from a different angle. It is not a guideline, but a response to one—a clinician’s reflection on what the AASM document omits, and what a more operational framework might look like, one which considers how central apnea physiology is a spectrum rather than a singular diagnosis, and which includes the patient as an active agent in decision-making. My proposal is deceptively simple: before prescribing any therapy for CSA, ask “why?”. Why are we treating this physiology? What is the goal of stabilizing this oscillating pattern?
Once that decision is made (the “why?” of therapy) it becomes easier to consider the many factors that can contribute to breathing instability—this leads to an inevitable discussion about the things that can be done to stabilize it.
BUT: lest we forget--It all starts with the “WHY?”.
And my favorite tool to explore the WHY? is the Five Reasons to Treat.²
But as usual, I’m getting ahead of myself…let me circle back and say more GOOD things about the new guidelines.
Walk with me…
What the Guideline Got Right
Let’s start with more words of credit!
The 2025 guideline represents a major methodological leap from its 2012 predecessor. It uses the full GRADE process, evaluates patient-important outcomes rather than surrogate physiology markers alone, and acknowledges heterogeneity across etiologies. It moves CSA a little closer to being understood not only as distinct entities (e.g.: “CHF induced Cheyne-Stokes” or “opioid-induced central apnea”) but as a spectrum of ventilatory control instability with many possible triggers.
It reviews all the familiar therapies: CPAP, still widely used but often failing to resolve events; BPAP, helpful with a backup rate for those with hypoventilation, but risky without; ASV, powerful at suppressing events but haunted by SERVE-HF and absent mortality benefit; oxygen, simple and effective at improving AHI but inconsistent in daytime outcomes; acetazolamide, modest but safe, especially short term for high-altitude CSA; and diaphragmatic pacing, promising but costly and with limited long-term data.
The guideline is clear about what it knows and what it does not know. It acknowledges physiologic diversity, concedes equifinality (a great word which means that CSA can be the final common result of a number of different pathophysiologic journeys), and highlights the need for more patient-reported outcomes. It closes, essentially, with a research agenda.
On paper, it is excellent.
In practice, I’m afraid it leaves many of us standing in the same old knot, wondering how to explain this hot mess to ourselves and to our patients.
We become the behavior we rehearse…
~ ~ ~ ~ ~
Device Deficiency Syndrome
In my clinic, I have seen the scenario play out countless times.
A patient is labeled with “Treatment-Emergent CSA.” The label itself triggers a cascade: This is TECSA, therefore CPAP won’t work, therefore we escalate to ASV. DING! DING! DING! DING! A $5,000 device is delivered. The patient is swept away by the jargon, the insurance company agrees to cover it (with a hefty deductible owed by the patient), and somewhere along the way no one has paused to ask: what are we trying to fix?
WHY ARE WE DOING THIS?
This is not malice; it is structure.
Our professional culture has trained us to treat the label, not the physiology, because it feels efficient. Guidelines reinforce this by presenting therapies in bins that match ICD codes. The problem is not that the therapies are ineffective—they are often very effective at suppressing events—but that the logic chain skips the essential question.
The skipped question is WHY.
The Five Reasons to Treat
Every therapy in medicine must justify itself. Why are we treating this patient? What are we trying to accomplish? In the Empowered Sleep Apnea project, we’ve distilled this into a simple collaborative complexity sensemaking mnemonic: the Five Reasons to Treat (FReTT).²
RISK – To reduce the incremental mortality risk that Sleep Apnea carries
SNORING – To relieve disruptive noise and vibrational trauma for the patient (and for the bed partner)
SLEEP – To improve sleep continuity, depth, duration and architecture
WAKE – To improve daytime function, cognition, and mood
COMORBIDITIES – To mitigate or manage interactions with vulnerable systemic diseases
Here’s the humane context: when we present FReTT to patients or providers, something shifts. The knot loosens. Instead of marching toward a DEVICE as a foregone conclusion, we pause to articulate the shared goal. Maybe the patient doesn’t care about cardiovascular risk, but desperately wants to stop waking up every hour. Maybe the partner is miserable from the snoring. Maybe the patient wants sharper cognition at work.
FReTT is not a replacement for evidence; it is the missing operational layer. It is the front door that the guideline does not provide.
CSA and the Mirage of RISK
Let us linger on the first of our Reasons to Treat: RISK.
Obstructive sleep apnea (OSA) has long been framed as a RISK-promoting scenario: untreated OSA increases the risk of hypertension, atrial fibrillation, motor vehicle accidents, strokes, cardiovascular death, and all-cause mortality. Treat OSA, the narrative goes, and you reduce the impact of that RISK on the patient’s life.
But CSA is different. The 2025 guideline admits as much, often between the lines. The natural history of primary CSA does not carry the signal for early demise, the same way OSA does. It follows that an event rate fueled primarily by central mechanism events should be decoded with this in mind. Trials of CPAP, ASV, and oxygen have shown reliable event suppression, sometimes improvements in LVEF, but no consistent mortality benefit. The SERVE-HF trial even suggested harm in a subset of patients treated with ASV, further shadowing the “we’re doing this for your longevity” narrative.
All this adds up to the idea the traditional “RISK” rationale to deploy treatment aimed to stabilize a CSA breathing pattern is shaky at best. We cannot honestly tell patients that stabilizing central apnea will prolong their life, particularly if the patient has no underlying heart disease.
And so, if RISK is not the dominant reason, the other four reasons rise in importance. CSA stabilization is primarily about SLEEP, WAKE, SNORING, and COMORBIDITIES. It is about quality of life. It is about stability. It is about partnership.
And this is where the “Many Moving Parts” discussion takes off.
Central Apnea Physiology—Many Moving Parts
This section is where I part ways most sharply with the guideline. While it acknowledges jargon-y contributors to CSA like loop gain, hypocapnic thresholds, and circulation time, it still organizes the clinical approach by labels and specific treatments, rather than breaking it down to actionable interventions on the understood underlying physiology.
In short, this guideline simply doesn’t provide guidance for how to look under the hood, and tinker—arguably a foundational component of patient-centered medicine.
See—in the “real world”, central apnea physiology is a moving target with many potentially “tweakable” moving parts, and it often co-exists with a whole spectrum of obstructive pathology events (OSA). Remember: the presence of central apnea physiology events does not preclude one’s responsibilities for managing obstructive pathology! This is no small detail: it’s in the overlap that the complexity reaches its “hot mess” zenith.
Interested?
Let’s take a short tour of this perplexing landscape…
…cue theremin sound effects as we scoot through alternate universes at dizzying speed…
The Many Universes of Central Apnea Physiology
In Black Hawk, Colorado (elevation 10,000 ft above sea level), a patient’s response to CPAP is unstable because of altitude-driven hypocapnia—he is told that he has TECSA...
…but, in an alternate universe that same patient living in Los Angeles, California, has a perfectly stable response…
…in another universe, he starts PAP using AutoPAP 5–20…the AHI response is unstable…he has prominent oral air venting …he doesn’t enjoy sleeping with the device…he is told that he has TECSA…
…in an alternate universe, he initiates PAP using AutoPAP 5–7 on a nasal mask and he enjoys a stable result…
…in yet another universe, the patient above is given an oronasal mask…he breathes with his mouth open…instead of a favorable response, the AHI response here is unstable…the patient reports discomfort exhaling against the pressure…
… when expiratory pressure relief (EPR) is added, the hypocapnia worsens, leading to worsening event instability…the patient is told he has TECSA…
…in an alternate universe, our patient from Los Angeles has atrial fibrillation, the circulation time stretches, feeding worsening oscillation….the event rate on PAP is unstable…the patient is told he has TECSA…
…in yet another universe, the patient in example #2 is a combat vet with PTSD--the arousal threshold plummets, amplifying instability….his result is unstable…the patient is told he has TECSA…
…in the universe in the dimension just next door, the patient has bad reflux treated for years with a proton pump inhibitor…he’s developed iron deficiency due to achlorhydria…as a result he has frequent periodic limb movements of sleep, and every jerk becomes a trigger for breathing overshoot…his response to CPAP is unstable…the patient is told he has TECSA…
WOWSERS!
When we look at the “CSA” conundrum from this lens…this “boots on the ground, real world management” lens, we can clearly see that it’s not a singular “disorder.” It’s a physiology—a manifestation of “many moving parts.”
A Gordian Knot.
And if it IS, in fact, a physiology (and it IS) then our job is not to treat the label but to parse the parts. Is this an iatrogenic hypocapnia problem? A hypoventilation problem? An arousal problem? An environmental hazard of living at altitude? A postural (open-mouth breathing) problem? An airway problem of obstructive events leading to frequent arousals leading to overcorrection? A non-airway problem, like periodic limb movements, provokikng sleep instability?
Considered this way, the label “G47.31” becomes a mere signpost, when it comes to therapy guidance—it points us in the right direction, but it still needs serious discussion.
Considered this way, we see instead a mystery to be solved, with each “moving part” meriting individual consideration…
~ ~ ~ ~ ~
Toward an Operational Framework
To break free of the “tyranny of labels”, the Empowered Sleep Apnea project proposes the following re-framing of CSA management:
Step 1: Name the “WHY?”
Which of the Five Reasons to Treat is motivating therapy? Are we aiming for RISK reduction (rarely), sleep continuity, daytime functioning, comorbidity management, or partner relief? The point here is that if no one can think of a reason to treat, it’s worth considering the notion that the situation may not require any treatment at all.
Step 2: Define the “WHAT?”
What do you want out of this? Define the measurable goals! Why do we want to stabilize the central events? Do we want to reduce the Epworth score? Do we want to have a positive impact on morning headaches? Do we want the partner to sleep through the night? Be precise and be specific. When following up, you’ll need to decide whether continuing the therapy is worth it. Know your reasons!
Step 3: Explore the “HOW?”
Map the physiology, and poke at it, with a stick:
Hypocapnia-driven effort instability
Use PAP to overcome obstructive component that triggers unstable breathing
Down-explore absolute pressures on PAP therapy to identify lowest effective pressure to overcome the obstructive pathology
Make nasal breathing a priority (mouth-breathing makes this instability worse!)—a nasal mask should be considered mandatory
Control oral air venting, assertively
Disable expiratory pressure relief (EPR)
Avoid bilevel PAP if there is no evidence of hypoventilation
Supplemental oxygen bled through device can have a stabilizing effect on CSA
Altitude dwellers: consider re-location to sea level (or try supplemental O2)
Hypoventilation-driven CSA (elevated CO2)—eg: CSA associated with chronic opiates, neuromuscular weakness, obesity/hypoventilation syndrome
consider ASV
consider BiLevel PAP with backup rate
consider diaphragmatic pacing
Long Circulation Time: (e.g.: atrial fibrillation, systolic heart failure): consider cardioversion to NSR, consider medical optimization of CHF, consider diaphragmatic pacing
Arousal threshold abnormalities (PTSD, PLMS, insomnia) → treat the arousal source. Meditative/functional breathing practices, hypnotic pharmacotherapy. Nasal breathing emphasis helps balance parasympathetic vs sympathetic tone, so the nasal mask we mentioned above is a good idea here, too.
Bridging Evidence and Practice
The guideline is not wrong—it is simply incomplete and lacking cohesion for boots-on-the-ground problem-solving. Its GRADE tables tell us what therapies can do. But they do not tell us what therapy is for, a pre-requisite step if our goal is to have an honest and transparent risk/benefit discussion about a particular treatment option, or how to work our way through them.
FReTT fills that gap. It gives clinicians and patients a shared language for goals. It prevents the human impulse to consider these diagnoses as “device deficiency syndromes.” It reframes CSA not as an enemy to be eradicated, but as a physiology to be stabilized in service of a larger aim.
~ ~ ~ ~ ~
A Story from the Field
Consider a patient with the following real-life scenario: Paul was labeled with “TECSA” after a CPAP titration with an oronasal mask demonstrated an unstable response at all pressures tested. His central apnea index was high. The recommendation was to return to the lab (another $1000 test) to trial ASV (a machine that costs much more than a standard CPAP).
When I asked Paul why he wanted treatment, he said: “I just want to sleep through the night without waking up every hour.” Lisa, his wife, added: “And I want the snoring gone.”
We decided to move ahead with CPAP using a nasal mask, a restricted pressure range, and assertive oral air venting management. As we expected, this successfully addressed the obstructive component of his problem (wife happy? Check!). His residual event rate at his home in the mountains (10,000 ft elevation) was high—AHI results on the device running anywhere from 5-30!
Interestingly, whenever he was visiting relatives in Los Angeles, his event rate on the device normalized…
After finding a gently-used oxygen concentrator on Craig’s List, we decided to move ahead with a trial running supplemental oxygen through his device, when he was sleeping in the mountains. The result was dramatic and seemingly miraculous: just like when he was in Los Angeles, with the supplemental oxygen, the event rate quieted down.
At last! The breathing during sleep stabilized, the patient slept through the night. The partner was relieved. The expensive (and sometimes over-pressurizing) ASV device was never needed.
The guideline would have called this “TECSA,” because he technically met the qualifications for it.
Yes, this was “technically” TECSA. But: was it?
If we followed the guidelines, we might never have explored the possibility that the oronasal mask was part of the problem…
…we might never have down-explored his pressure…
…if we were still suffering from the DEVICE-DEFICIENCY DELUSION, we might have gone ahead and slapped his oronasal mask-encrusted face with an ASV device, which (in this scenario) would almost certainly give him an even more unstable result--dooming him to consider more invasive and expensive options...
…only now he’s got a new label, with even deeper consequences, therapy wise… that label being…
“CPAP intolerant.”
~ ~ ~ ~ ~
Conclusion: Untying the Knot
The 2025 AASM guideline is a worthy, rigorous document. It reviews evidence, balances harms and benefits, and acknowledges uncertainty. But for me, it leaves out the most important question: why are we treating?
If CSA stabilization is not primarily about RISK (and it isn’t)—it’s the other four reasons we have to contend with: snoring relief, sleep continuity, wake function, and comorbidity management.
By naming the WHY (FReTT), the WHAT (measurable goals), and the HOW (central apnea physiology, rather than label-based, DEVICE-oriented care), clinicians can move beyond cultural “DEVICE-DEFICIENCY” reflexes and into true patient-centered care.
CSA is not a singular disease, folks. It’s a complex physiology that has been conveniently tied up into a knot within our system so that we can talk about it. There’s a whole lot more there, underneath the package, though. We owe it to our patients to dig deeper.
Our job here is not to swallow the solidarity that our tidy little knot would suggest…but to untie it carefully, patiently, with the patient’s goals at the center.
With all that being said, I figured I’d close this missive with a DAD JOKE…so…um…hang onto your eye-sockets…this one’s a doozy:
NOT DAD: Hey! Central apnea! Are you a “singular being” with a “one-size-fits-all” solution?
DAD: NOPE! I’m a frayed knot!
(tee hee!)
Cue pool-ball-clicking sound effects as eyes roll collectively backwards…clink!clink!
Kind mojo,
Dave
David E McCarty MD FAASM
Boulder, Colorado
References
American Academy of Sleep Medicine. Treatment of Central Sleep Apnea in Adults: Clinical Practice Guideline. Darien, IL: AASM; 2025.
McCarty DE, Stothard E. Empowered Sleep Apnea: A Handbook for Patients and the People Who Care About Them. Pennsauken, NJ: BookBaby Press; 2022.